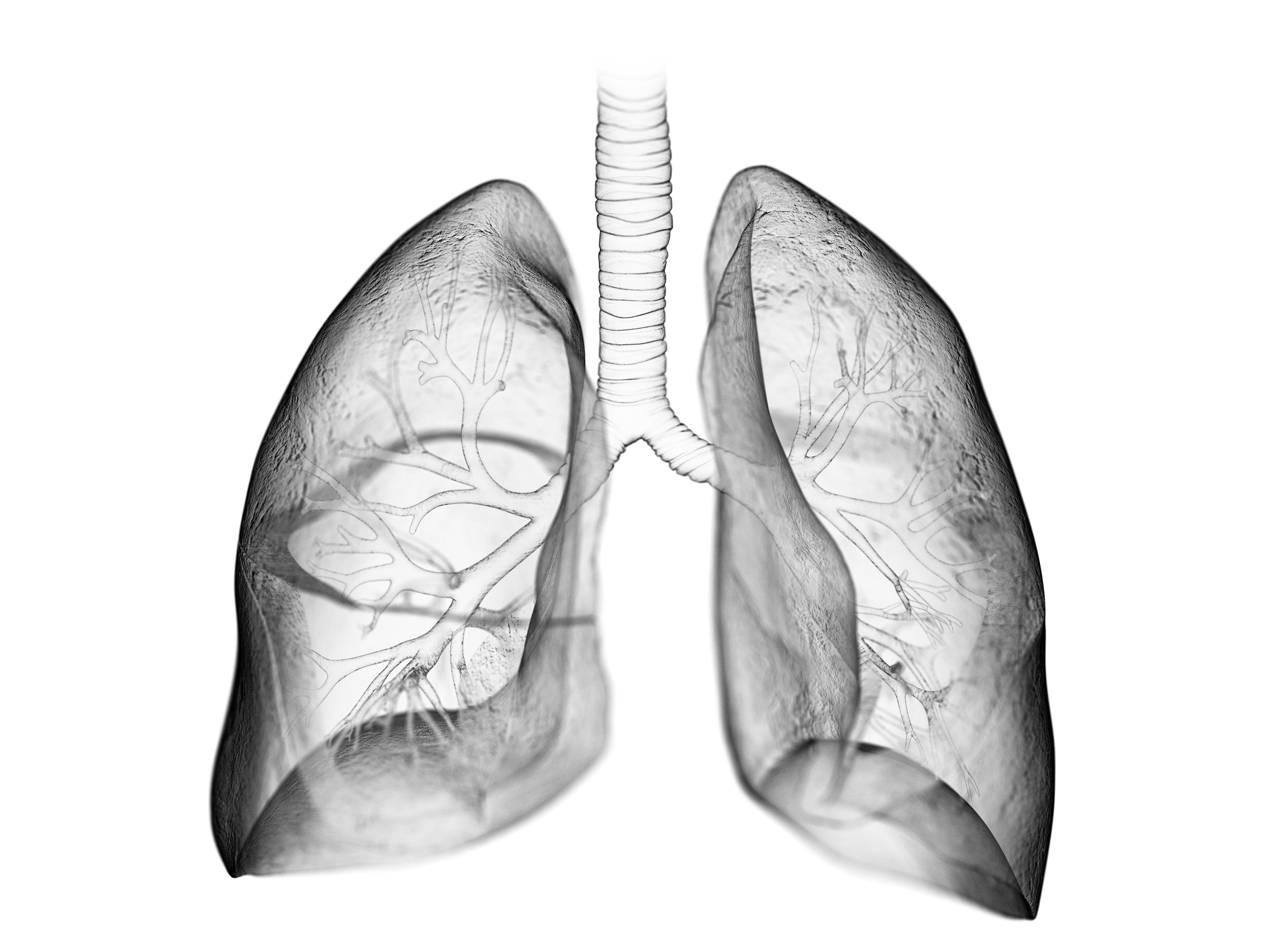
Lung and Kidney Development
Morphogenesis, the process of organ assembly, relies on complex interactions - physical, chemical, and electrical - between multicellular populations of cells. Dysregulation of this process leads to a variety of congenital birth defects that can lead to mortality or life long complications. Much of the work to date has focused on genetic and molecular signals that drive these processes; however, we seek to understand how mechanical forces interact with cellular signaling networks to sculpt the physical process of morphogenesis. Studying developing tissues is incredibly difficult due their 3D nature and the need to visualize these dynamic mechanical environments over time. In our lab, we are building systems that allow us to visualize and perturb the cellular mechanical environments to understand the role of these forces in creating tissues. Specifically we are investigating the role of the extracellular matrix (ECM), tissue strains, tissue stiffness, and the role of fluid flows in driving cellular behaviors and organ formation to inform clinical treatment.
CR Gonyea, Y Shen, KM Nelson, R Bird, RM Gilbert, OO Olutoye, S Keswani, JP Gleghorn (2025) The nitrofen/bisdiamine murine model of congenital diaphragmatic hernia has a pulmonary hypertension vascular phenotype consistent with human CDH, AJP-Lung, 329:1, L48-L60
Shen, Y., Gleghorn, J.P. (2025) Class III Phosphatidylinositol-3 Kinase/Vacuolar Protein Sorting 34 in Cardiovascular Health and Disease. J. Cardiovasc. Transl. Res. 18, 392–407
S Mohr-Allen, JP Gleghorn, VD Varner. Local changes in luminal fluid pressure alter proliferation and lateral branching morphogenesis within the embryonic avian lung. Developmental Biology, 520, 251-263
S Chakraborty, KE Peak, JP Gleghorn, TJ Carroll, VD Varner. (2024) Quantifying spatial patterns of tissue stiffness within the embryonic mouse kidney. Methods in Molecular Biology, 2805:171-186.
K Lingappan, OO Olutoye, A Cantu, MC Gutierrez, N Cortes-Santiago, JD Hammond, J Gilley, F Polverino, JR Quintero, H Li, JP Gleghorn, SG Keswani. (2023) Molecular insights using spatial transcriptomics of the distal lung in congenital diaphragmatic hernia. AJP-Lung. 325(4), L477-L486
RM Gilbert, JP Gleghorn. (2023) Congenital diaphragmatic hernia is caused by environmental and genetic factors related to vitamin A deficiency. American Journal of Physiology - Lung Cellular and Molecular Physiology, 324(4):L456-67.
B Hayward-Piatkovskyi, CR Gonyea, SC Pyle, K Lingappan, JP Gleghorn. (2023) Sex-related external factors influence pulmonary vascular angiogenesis in a sex-dependent manner. American Journal of Physiology - Heart and Circulatory Physiology, 324(1):H26-32.
KE Peak, SR Mohr-Allen, JP Gleghorn, VD Varner. (2022) Focal sources of FGF-10 promote the buckling morphogenesis of the embryonic airway epithelium. Biology Open, 11(9):bio059436.
OO Olutoye II, WD Short, J Gilley, JD Hammond II, MA Belfort, TC Lee, A King, J Espinoza, L Joyeux, K Lingappan, JP Gleghorn, SG Keswani. (2022) The cellular and molecular effects of fetoscopic endoluminal tracheal occulsion in congenital diaphragmatic hernia, Frontiers in Pediatrics, 10:925106.
RM Gilbert, LE Schappell, JP Gleghorn. (2021) Defective mesothelium and limited physical space are drivers of dysregulated lung development in a genetic model of congenital diaphragmatic hernia. Development, 148(10).
LE Schappell, DM Minahan, JP Gleghorn. (2020) A microfluidic system to measure neonatal lung compliance over late stage development as a functional measure of lung tissue mechanics. Journal of Biomechanical Engineering, 142(10):100803.
JT Morgan*, WG Stewart*, RA McKee, JP Gleghorn. (2018) The mechanosensitive ion channel TRPV4 is a regulator of lung development and pulmonary vascular stabilization. Cellular and Molecular Bioengineering, 11(5): 309-320. *denotes authors contributed equally
CM Nelson, JP Gleghorn, MF Pang, VD Varner, E Miller, DC Radisky, HA Stone. (2017) Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development, 144(23): 4328-4335.